
Lacerta Tokyo Сo.,Ltd.
We aim for active open innovation for developing new products and technology,
meeting the needs of practitioners and customers.

PDRN
PEPTIDES
BOTULINUM TOXIN TYPE A
Peptides are a family of substances in which molecules are built from 2 or more amino acids. Peptides contain about half of all known hormones and most enzymes. But there is also a special class of compounds — peptide biomodulators. They differ from other peptides in their ability to induce protein synthesis.
Peptide bioregulators is a prominent gerontologist, Professor, MD V.H.It was discovered in the 70s by Havinson. Therefore, they are called Havinson peptides.
Where do Havinson Peptides come from in Cells
The protein synthesized in the cell does its job, after which it is destroyed. With the help of peptidase enzymes, it is “cut” into fragments. Some of them leave the body through the excretory system. And the other parts are fragmented. Some protein fragments contain segments of amino acids connected in a certain way. These are the peptide bioregulators that Havinson discovered.
Biomodulators strictly correspond to certain parts of the DNA molecule. It is mapped to this area like a magnetic key that gives a signal to the magnetic lock to open the door. DNA molecules are unwound, from which information is read, a cheat sheet matrix (RNA) is built, and proteins are synthesized according to it – those that were disposed of before. Thus, biomodulatory peptides are formed from proteins, but without them the synthesis of the same proteins is impossible.
Why Do I Need to Take Peptide Biomodulators
Even in ideal conditions, a healthy body, with the help of internal reserves, makes up only 90% of the deficiency of peptides, and the remaining 10% with food.
Receive together.This problem begins at the moment when stressors begin to act on the body:
– Sedentary work;
– stuffy room.;
– Improper nutrition;
– poor ecosystem.,
-Other
Under their influence, organs and tissues gradually wear out and no longer have enough peptides that come with food. In this case, peptide drugs come to the rescue, which temporarily compensate for this deficiency.
In the future, when the tissue produces the right amount of the necessary protein, its function normalizes. However, when the body is subjected to stress loads, as before, the work of organs and tissues again fails and again needs support.
How Havinson peptides work and how to take them: we will tell you in simple words
Biocomodulators affect only the organs in which they are isolated. For example, Endoluten acts on the epiphysis, and Visoluten acts on the eyes. Therefore, the doctor can prescribe a course of several drugs with different peptides in the composition. But experts do not recommend taking more than 5 biomodulators at the same time.
First of all, it is necessary to influence the mechanisms of adaptation to stress and put in place the detoxification system. Therefore, it is recommended to start treatment with drugs to restore the neuroendocrine, immune, vascular and nervous systems and normalize the work of other systems and organs with the help of local peptides, such as liver, cartilage, blood vessels.
Peptide drugs have a cumulative effect – each subsequent course is more effective than the previous one. After a 1-3 month course of peptides, they act for another 6 months. Therefore, in order to maintain the work of the organ at the proper level, it is recommended to take 1 course of peptide biomodulators in 2 years.
Peptide biomodulators are not only compatible with each other, but also with traditional medicines. Thanks to their reception, it is possible to minimize the dose of the main remedy – of course, after examination and consultation with the attending physician.
Adaptation
It makes sense to take peptide bioregulators for all people starting from the age of 20. They are also affected by people who live or work in conditions of increased stress and poor environmental conditions: residents of large cities, employees of industrial enterprises, office employees.
Types and classifications of peptide biomodulators
There are 38 peptide bioregulators: natural animal raw materials are used in the manufacture, since all mammals have the same peptide bioregulators. 17 Artificial – they are made from amino acids isolated from plants. An artificial peptide biomodulator is a shortened copy of the main peptide contained in the extract. Their effect does not last as long as the natural one, but it is achieved faster. This is due to the fact that the molecules are short and their concentration is much higher. According to Professor Havinson, artificial peptides will become indispensable when humanity feels a lack of natural ones.
Preparations with peptide biomodulators can be divided into 3 groups:
Cytomax is a natural Havinson peptide. In the capsule of each organ there is a separate drug. Some of them have the form of drops under the tongue — a series of “tongues”. For external use, peptide complexes are also present in the solution. Each such complex contains peptides for a certain group of organs.
Cytogen is an artificial Habinson peptide in a capsule. Each preparation contains peptides for 1 particular organ.
Revilab – a peptide complex containing up to 4 artificial peptides of different organs. There are 2 types of Revilab SL— drips under the tongue and Revilab ML—capsules.
Peptides for the repair of the central nervous system and brain
Havinson peptides are especially relevant in adulthood. Clinically proven: bioregulators restore so-called spikes that stimulate the neural network. Therefore, taking them not only increases life expectancy, but also prevents the development of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease.
Other Important Things to Know about Peptides: Answers to Frequently Asked Questions
Why can I take peptide biomodulators orally? Are they not destroyed in the stomach?
Havinson peptides are able to maintain their structure when absorbed into the gastrointestinal tract. This is possible due to the fact that they are very short: their length is no more than 20 amino acids, and the active site consists only of 2-3 amino acids. Such a small structure does not disintegrate in the digestive system and easily penetrates into the cell membrane. The effectiveness of the drug in capsules is confirmed by clinical studies, in addition to being much more convenient than injections.
Can I take multiple peptide drugs at the same time?
Since peptides act in a targeted manner, several drugs can be used simultaneously. They are compatible with each other, do not interfere with each other and are combined with other drugs, including medicinal drugs. Exception: It is not recommended to take Glandokort (adrenal peptide) and Endoluten (epiphyseal peptide) at the same time.
What happens if you stop taking peptide bioregulators? Do you have withdrawal syndrome?
Peptides activate DNA, thereby triggering protein synthesis in cells, which has already happened, but are not aggressive enough due to stress or peptide deficiency. So, there is no withdrawal syndrome, and the worst thing that can happen is that after the disappearance of additional peptides, the cells return to the previous level of synthesis. But, as practice shows, it will be higher than the first. Havinson peptides are not hormones, they have a different mechanism of action and there is no withdrawal syndrome. Taking thyroid bioregulators does not wean the thyroid gland to function, but only helps it naturally produce the right amount of hormones.
Does the peptide cause allergies, mutations, oncological diseases?
Peptide biomodulators are not foreign substances and are not rejected by the body. The new generation of peptides developed by the Bio-Regulatory Institute and the peptide company are well purified and do not contain phospholipids, which are residues of cell membranes. They are not foreign antigens and do not produce an immune response. Since peptides cause only natural processes of protein synthesis (specific to cells), there are no allergic reactions and no mutations occur.
Is it possible to overdose on peptides?
Overdose can not be – excess peptides are simply excreted through the kidneys or intestines. In the study, experiments were conducted in mice — the medicinal amount of pancreatic peptides exceeded 10 million times. There was no result.
Peptides are a family of substances whose molecules are built from two or more amino acids. Peptides include about half of all known hormones and most enzymes. But there is also a special class of compounds — peptide bioregulators. They differ from other peptides in their ability to trigger protein synthesis.
Peptide bioregulators were discovered in the 1970s by an outstanding gerontologist, Professor, MD V.H. Havinson. Therefore, they are called Havinson peptides.
Where do Havinson peptides come from in cells
The protein that was synthesized in the cell does its job and after that it is destroyed. With the help of peptidase enzymes, it is “cut” into fragments. Some of them leave the body through the excretory system. And the other part is fragmented. Some protein fragments contain segments of amino acids connected in a certain way. These are the peptide bioregulators that Havinson discovered.
The bioregulator strictly corresponds to a certain section of the DNA molecule. It is mapped to this area like a magnetic key, which gives a signal to the magnetic lock to open the door. The DNA molecule is unwound, information is read from it, and cheat sheet matrices (RNA) are built, according to which a protein will be synthesized – the one that was disposed of before. Accordingly, bioregulatory peptides are formed from protein, but without them the synthesis of the same protein is impossible.
Why do I need to take peptide bioregulators
Even in ideal conditions, a healthy body, with the help of internal reserves, makes up for the deficiency of peptides by only 90%, and receives the remaining 10% with food. The problem begins at the moment when stress factors begin to act on the body:
– sedentary work;
– stuffy rooms;
– improper nutrition;
– poor ecology,
– etc.
Under their influence, organs and tissues gradually wear out and they no longer have enough peptides that come with food. In this case, peptide drugs come to the rescue, which temporarily make up for this deficiency.
In the future, when the tissue produces the right amount of necessary proteins, its function will normalize. But if the body is subjected to stress loads as before, the work of organs and tissues will fail again, and they will need support again.
How Havinson peptides work and how to take them: we tell you in simple words
Bioregulators affect only the organ from which they were isolated. For example, Endoluten will act on the epiphysis, and Visoluten on the eyes. Therefore, doctors can prescribe a course of several drugs with different peptides in the composition. But experts do not recommend taking more than five bioregulators at the same time.
First of all, it is necessary to influence the mechanism of adaptation to stress and put the detoxification system in order. Therefore, we recommend starting therapy with drugs to restore the neuroendocrine, immune, vascular and nervous systems, and then normalize the work of other systems and organs with the help of local peptides: liver, cartilage, blood vessels, etc.
Peptide drugs have a cumulative effect — each subsequent course will be more effective than the previous one. After a 1-3 month course of peptides, they act for another six months. Therefore, in order to maintain the work of organs at the proper level, it is advisable to take two courses of peptide bioregulators per year.
Peptide bioregulators are compatible not only with each other, but also with traditional medicines. Thanks to their reception, it is possible to minimize the dosage of the main therapeutic drug – of course, after examination and consultation with the attending physician.
Indications
It makes sense to take peptide bioregulators for all people starting from the age of 20. They should also be taken by people who live or work in conditions of increased stress and poor environmental conditions: residents of megacities, employees of industrial enterprises, office employees.
Types and classification of peptide bioregulators
There are 38 peptide bioregulators:
21 natural — animal raw materials are used for production, since all mammals have the same peptide bioregulators. 17 artificial — they are made from amino acids isolated from plants. Artificial peptide bioregulators are shortened copies of the main peptide that is included in the extract. Their effect is not as long-lasting as natural ones, but it is achieved faster. This is because the molecules are shorter and their concentration is much higher. According to Professor Havinson, artificial peptides will become indispensable when humanity feels a shortage of natural ones.
Preparations with peptide bioregulators can be divided into 3 groups:
Cytomax are natural Havinson peptides. There is a separate drug in capsules for each organ. Some of them are in the form of drops under the tongue — a series of “Lingual”. There are also peptide complexes in solution, for external use. Each such complex contains peptides for a specific group of organs.
Cytogens are artificial Havinson peptides in capsules. Each preparation contains peptides for one specific organ.
Revilab — peptide complexes containing up to 4 artificial peptides of different organs. There are two types: Revilab SL — drops under the tongue, and Revilab ML — capsules.
Peptides for the restoration of the central nervous system and brain
Havinson peptides are especially relevant in adulthood. Clinically proven: bioregulators restore the so-called spikes that stimulate the neural network. Therefore, taking them not only increases life expectancy, but also prevents the development of neurodegenerative diseases – Alzheimer’s, Parkinson’s and others.
What else is important to know about peptides: answers to frequently asked questions
Why can peptide bioregulators be taken orally? Are they not destroyed in the stomach?
Havinson peptides are able to maintain their structure when absorbed in the gastrointestinal tract. This is possible due to the fact that they are very short: their length is no more than 20 amino acids, and the active site consists of only 2-3 amino acids. Such small structures do not disintegrate in the digestive system and easily penetrate the cell membrane.
The effectiveness of drugs in capsules has been confirmed by clinical studies, besides they are much more convenient than injections.
Can I take several peptide medications at the same time?
Since peptides act in a targeted manner, several drugs can be used simultaneously. They are compatible with each other, do not interfere with each other and are combined with other drugs, including medicinal ones. Exception: it is not recommended to take Glandocort (adrenal peptides) and Endoluten (epiphysis peptides) at the same time.
What will happen if you stop taking peptide bioregulators? Will there be a withdrawal syndrome?
Peptides activate DNA and thereby trigger protein synthesis inside the cell, which is already going on, just not actively enough due to stress or peptide deficiency. Therefore, there will be no withdrawal syndrome and the worst that can happen is that after the disappearance of additional peptides, the cell will return to its previous level of synthesis. But, as practice shows, it will be higher than the initial one.
Havinson peptides are not hormones, they have a different mechanism of action and there is no withdrawal syndrome. Taking a thyroid bioregulator, you do not wean the thyroid gland to work, you only help it to create hormones naturally and in the right amount.
Do peptides cause allergies, mutations and oncological diseases?
Peptide bioregulators are not foreign substances and they are not rejected by the body. The new generation peptides developed by the Institute of Bioregulation and Peptides company are well purified, they do not contain phospholipids — remnants of cell membranes. They are not foreign antigens, they will not produce an immune response. There will be no allergic reactions, mutations will not occur, since peptides trigger only the natural process of protein synthesis — the one that is peculiar to the cell.
Is an overdose of peptides possible?
There can be no overdose — excess peptides will simply be excreted through the kidneys or intestines. In the study, an experiment was conducted on mice — the medicinal dose of pancreatic peptides was exceeded by 100 thousand times. There were no consequences.
Peptide bioregulators is a prominent gerontologist, Professor, MD V.H.It was discovered in the 70s by Havinson. Therefore, they are called Havinson peptides.
Where do Havinson Peptides come from in Cells
The protein synthesized in the cell does its job, after which it is destroyed. With the help of peptidase enzymes, it is “cut” into fragments. Some of them leave the body through the excretory system. And the other parts are fragmented. Some protein fragments contain segments of amino acids connected in a certain way. These are the peptide bioregulators that Havinson discovered.
Biomodulators strictly correspond to certain parts of the DNA molecule. It is mapped to this area like a magnetic key that gives a signal to the magnetic lock to open the door. DNA molecules are unwound, from which information is read, a cheat sheet matrix (RNA) is built, and proteins are synthesized according to it – those that were disposed of before. Thus, biomodulatory peptides are formed from proteins, but without them the synthesis of the same proteins is impossible.
Why Do I Need to Take Peptide Biomodulators
Even in ideal conditions, a healthy body, with the help of internal reserves, makes up only 90% of the deficiency of peptides, and the remaining 10% with food.
Receive together.This problem begins at the moment when stressors begin to act on the body:
– Sedentary work;
– stuffy room.;
– Improper nutrition;
– poor ecosystem.,
-Other
Under their influence, organs and tissues gradually wear out and no longer have enough peptides that come with food. In this case, peptide drugs come to the rescue, which temporarily compensate for this deficiency.
In the future, when the tissue produces the right amount of the necessary protein, its function normalizes. However, when the body is subjected to stress loads, as before, the work of organs and tissues again fails and again needs support.
How Havinson peptides work and how to take them: we will tell you in simple words
Biocomodulators affect only the organs in which they are isolated. For example, Endoluten acts on the epiphysis, and Visoluten acts on the eyes. Therefore, the doctor can prescribe a course of several drugs with different peptides in the composition. But experts do not recommend taking more than 5 biomodulators at the same time.
First of all, it is necessary to influence the mechanisms of adaptation to stress and put in place the detoxification system. Therefore, it is recommended to start treatment with drugs to restore the neuroendocrine, immune, vascular and nervous systems and normalize the work of other systems and organs with the help of local peptides, such as liver, cartilage, blood vessels.
Peptide drugs have a cumulative effect – each subsequent course is more effective than the previous one. After a 1-3 month course of peptides, they act for another 6 months. Therefore, in order to maintain the work of the organ at the proper level, it is recommended to take 1 course of peptide biomodulators in 2 years.
Peptide biomodulators are not only compatible with each other, but also with traditional medicines. Thanks to their reception, it is possible to minimize the dose of the main remedy – of course, after examination and consultation with the attending physician.
Adaptation
It makes sense to take peptide bioregulators for all people starting from the age of 20. They are also affected by people who live or work in conditions of increased stress and poor environmental conditions: residents of large cities, employees of industrial enterprises, office employees.
Types and classifications of peptide biomodulators
There are 38 peptide bioregulators: natural animal raw materials are used in the manufacture, since all mammals have the same peptide bioregulators. 17 Artificial – they are made from amino acids isolated from plants. An artificial peptide biomodulator is a shortened copy of the main peptide contained in the extract. Their effect does not last as long as the natural one, but it is achieved faster. This is due to the fact that the molecules are short and their concentration is much higher. According to Professor Havinson, artificial peptides will become indispensable when humanity feels a lack of natural ones.
Preparations with peptide biomodulators can be divided into 3 groups:
Cytomax is a natural Havinson peptide. In the capsule of each organ there is a separate drug. Some of them have the form of drops under the tongue — a series of “tongues”. For external use, peptide complexes are also present in the solution. Each such complex contains peptides for a certain group of organs.
Cytogen is an artificial Habinson peptide in a capsule. Each preparation contains peptides for 1 particular organ.
Revilab – a peptide complex containing up to 4 artificial peptides of different organs. There are 2 types of Revilab SL— drips under the tongue and Revilab ML—capsules.
Peptides for the repair of the central nervous system and brain
Havinson peptides are especially relevant in adulthood. Clinically proven: bioregulators restore so-called spikes that stimulate the neural network. Therefore, taking them not only increases life expectancy, but also prevents the development of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease.
Other Important Things to Know about Peptides: Answers to Frequently Asked Questions
Why can I take peptide biomodulators orally? Are they not destroyed in the stomach?
Havinson peptides are able to maintain their structure when absorbed into the gastrointestinal tract. This is possible due to the fact that they are very short: their length is no more than 20 amino acids, and the active site consists only of 2-3 amino acids. Such a small structure does not disintegrate in the digestive system and easily penetrates into the cell membrane. The effectiveness of the drug in capsules is confirmed by clinical studies, in addition to being much more convenient than injections.
Can I take multiple peptide drugs at the same time?
Since peptides act in a targeted manner, several drugs can be used simultaneously. They are compatible with each other, do not interfere with each other and are combined with other drugs, including medicinal drugs. Exception: It is not recommended to take Glandokort (adrenal peptide) and Endoluten (epiphyseal peptide) at the same time.
What happens if you stop taking peptide bioregulators? Do you have withdrawal syndrome?
Peptides activate DNA, thereby triggering protein synthesis in cells, which has already happened, but are not aggressive enough due to stress or peptide deficiency. So, there is no withdrawal syndrome, and the worst thing that can happen is that after the disappearance of additional peptides, the cells return to the previous level of synthesis. But, as practice shows, it will be higher than the first. Havinson peptides are not hormones, they have a different mechanism of action and there is no withdrawal syndrome. Taking thyroid bioregulators does not wean the thyroid gland to function, but only helps it naturally produce the right amount of hormones.
Does the peptide cause allergies, mutations, oncological diseases?
Peptide biomodulators are not foreign substances and are not rejected by the body. The new generation of peptides developed by the Bio-Regulatory Institute and the peptide company are well purified and do not contain phospholipids, which are residues of cell membranes. They are not foreign antigens and do not produce an immune response. Since peptides cause only natural processes of protein synthesis (specific to cells), there are no allergic reactions and no mutations occur.
Is it possible to overdose on peptides?
Overdose can not be – excess peptides are simply excreted through the kidneys or intestines. In the study, experiments were conducted in mice — the medicinal amount of pancreatic peptides exceeded 10 million times. There was no result.
For many years, it was believed that aging begins with a cell. However, the root cause is deeper. Our whole body consists of tissues, and they, in turn, are only 20% cells and 80% extracellular matrix.
The destruction of the extracellular matrix is the main cause of aging.
What kind of matrix is this? This is a kind of binding medium for cells. It can fill the thin gaps between cells (for example, in the liver and muscles), and can fill the bulk of the space (for example, between the cells of connective, bone and cartilage tissue).
The extracellular matrix contains 3 main components:
– collagens;
– adhesive proteins (bind various components of the matrix, fix cells);
– proteoglycans (this is a key component, proteoglycans serve as a filler in the matrix and are its main substance).
Extracellular matrix functions:
– Forms the skeleton of tissues and organs;
– It has a direct effect on the development and metabolism of cells;
– It is a universal “biological glue”;
– Participates in the regulation of water-salt metabolism in the body;
– Forms some structures (cartilage, bones, tendons, teeth);
– Transmits signals from the regulatory systems of the body – to the cells. It is the extracellular matrix that controls neutrotransmitters – biologically active substances that transmit electrical impulses and provide information exchange in the body with the help of the nervous, cardiovascular, endocrine and immune systems.
Performs the role of a “molecular sieve”: nutrients and oxygen move from the blood to the cells through the matrix. The reverse process also occurs: cells through the matrix remove toxins, carbon dioxide and decay products (metabolites) into the blood;
Provides the movement of hormones from the blood to the cell receptors;
Provides movement of mediators from nerve endings.
Extracellular matrix and aging
The process of destruction of the extracellular matrix is not lightning fast. The first destructive changes usually begin at the age of 20, and sometimes even earlier.
How does the matrix change:
1. A healthy matrix is a homogeneous and uniform structure – sol. All matrix functions are active.
2. Under the influence of harmful factors, toxins accumulate in the matrix, and the pH shifts towards a more acidic environment. This causes swelling of the extracellular matrix (due to the attraction of water molecules to high-polymer sugars) and converts it into a gel state. The matrix becomes acidified, heterogeneous and compacted. The metabolism in the cells slows down, the access of oxygen and the removal of carbon dioxide is difficult.
3. Toxins form chemical bonds with sugars. Polymerization occurs with the structures of the extracellular matrix. Cells stop coping with the excretion of decay products and do not receive sufficient nutrition. The development of chronic diseases begins.
4. As toxins accumulate in the extracellular matrix, harmful components penetrate into the depth of the cell and affect structures in its cytoplasm – the protein content. For example, mitochondria are affected by toxins. As a result, the self-regulation (homeostasis) of the cell is critically disrupted: it becomes acidified. And as a result of oxygen starvation, anaerobic glycolysis is triggered in the cells instead of aerobic. What does this mean?
Aerobic glycolysis is the breakdown of glucose with the participation of oxygen, for the formation of energy.
Anaerobic glycolysis is the breakdown of glucose without oxygen. Occurs only in cells with hypoxia – oxygen starvation. As a result, lactate – lactic acid is formed.
5. A constant energy deficit in cells leads to the fact that the cell nucleus receives information: it is necessary to produce more mitochondria. This causes nonspecific cell division. The cell multiplies uncontrollably. And a malignant tumor is formed. Excess lactic acid begins to enter the extracellular matrix. And it is rebuilt so much that it becomes practically impervious to immune cells.
Many gerontologists (specialists in the study of the causes of aging of the body and ways to resist it) agree that oncological diseases are one of the signs of aging.
Destruction of the extracellular matrix is one of the proofs.
Is it possible to stop aging?
Knowledge of the mechanisms of aging of the body helps to prevent premature changes in tissues and organs. And now, when it has already been proven that the main reason is a violation of the structure of the extracellular matrix, everyone can, if they wish and with patience, improve their condition, start moving towards recovery and rejuvenation.
Be careful with all kinds of sugars. They lead to glycation/glycation – the main cause of accelerated aging. Glucose literally glues collagen together, forming so-called vertical “crosslinking” in the extracellular matrix.
The more such protein “crosslinking”, the less elastic the tissues become. The extracellular matrix turns into a rigid substance. At the same time, not only the skin suffers, but also the joints, capillaries, and retina of the eyes.
Is it true that a sweet tooth ages earlier?
There is no need to talk about fast carbohydrates: if you quench your thirst for sweets with the help of sweets, cakes, cakes and other similar desserts, then glycation goes at a rapid pace. Dried fruits and fresh fruits are already slow carbohydrates, however, their overabundance will lead to the formation of “crosslinking”.
Indulge yourself with healthy sweets in moderation.
Avoid excessive exposure to ultraviolet radiation. The desire to get a tan is a relatively new trend. A little over 100 years ago, any aristocrat hid her face from the sun’s rays under wide-brimmed hats and umbrellas. And now, for example, no sane Japanese woman will expose her skin to harmful radiation. Because any woman knows that nothing ages the skin like the sun.
It is enough to look at the so-called “triangle of old age” – the decollete area in the part where there is usually a cutout in the dress. The skin in this area wrinkles first of all, turning into a “chicken tail”. But that part of the chest, which is usually covered with a swimsuit / clothing, retains youth longer.
There is no need to talk about the face: the sun causes irreversible changes in collagen and elastin fibers, causes pigmentation and triggers the production of matrix metalloproteinases – enzymes that destroy matrix proteins (that is, they actually destroy collagen).
Avoid tobacco smoke as much as possible. And in general about being in a room where it is smoked. A study conducted at a research university in Germany in 2015 proved that tobacco smoke affects the body in the same way as estrogen deficiency: it worsens the metabolism in the skin, slows down wound healing, destroys all types of extracellular matrix proteins.
A low-calorie diet helps to reduce the rate of glycation. Long-lived collagen fibers are regularly replaced in the extracellular matrix: every 10 years. However, this does not apply to already glycated collagen.
Usually, at the age of 20, a person first appears “crosslinking” of collagen as a result of glycation. The volume of glycated collagen increases by 3.7% every year. By the age of 80, the number of “stitches” increases to 50%.
Glycation occurs not only as a result of excessive consumption of sweets. The end products of glycation accumulate in the body and because of a great love for fried, fatty.
Eating foods with a low glycemic index helps prevent premature aging and prolong youth.
Taking vitamin D prevents liver fibrosis. Fibrosis is the growth of connective tissue with the formation of scars. Fibroplasts and type I and type III collagen in the extracellular matrix take part in this process.
Taking vitamin D suppresses the formation of type I collagen and blocks the growth of connective tissue.
What is the danger of liver fibrosis? The fact that the body ceases to perform its main functions:
– Amino acid (protein metabolism). It is in the liver that proteins are formed that ensure blood clotting.
– Immune (protection against infectious and other harmful factors).
– Formation of protein complexes with fats, carbohydrates.
– Formation of complexes-carriers of valuable elements. For example, transferrin, which carries iron through the body.
– Cleavage of proteins to the final products – ammonia and urea (for subsequent excretion through the kidneys). At the same time, the liver neutralizes ammonia. But with fibrosis, the organ is no longer able to perform such a function, as a result, ammonia accumulates in the blood and can lead to serious disorders of the central nervous system, up to coma.
– Lipid (fat) metabolism. In the process of splitting fats in the liver, fatty and other acids are formed.
– Carbohydrate metabolism. Glycogen is synthesized and decomposed, fructose and galactose are converted into glucose, glucose itself is oxidized.
– Assimilation, storage and formation of vitamins.
– Detoxification of the body.
– Participation in the exchange of valuable elements (iron, copper and others).
– A clean liver is our youth, beauty and health in general.
Monitor your blood pressure. High blood pressure causes an increase in the level of the hormones angiotensin II and aldosterone. And they, in turn, stimulate fibrosis of tissues and organs. People with low blood pressure tend to age later than hypertensive patients.
Previously, I considered my low blood pressure (80/50, at best – 90/60) a real disaster: the feeling of weakness haunted almost all day, the immune system also often malfunctioned.
But hypotonics are actually easier: useful physical activity and wellness exercises help you feel much better. And age-related skin changes appear, indeed, later than in people suffering from high blood pressure.
Recommendations for hypertensive patients to lower blood pressure:
– deep breathing at a calm pace;
– acupressure (on the outside of the palm between the index and thumb);
– exhale air into an empty bottle through the neck: inhale through the nose, and exhale through the mouth;
– taking a hot shower for a minute;
– contrast foot baths;
– compress with apple cider vinegar on the feet (for 15-20 minutes);
– hypothetical drinks (teas from karkade and hawthorn, beet juice, cranberry juice);
– watermelons and persimmons.
Refusal of fried food. And it’s not about increasing the risk of obesity. The staff of Mount Sinai Medical School in the USA conducted a number of studies in 2008, changing the diet of mice. And they found that life expectancy increases only if the volume of glycation end products in food is reduced. Moreover, reducing the consumption of fast carbohydrates alone is not enough. An increase in life expectancy was noted only in those who were not given fried, baked food, as well as long-stored fats and fats of old animals.
The fact is that during the frying process, the volume of glycation end products increases at a record pace. Most of these harmful components are in fried bacon. Butter comes in second place. For example, 100 g of fried bacon contains as much carboxymethyllysine as our body is able to neutralize in a whole week at best.
The healthiest diet in order to consume as few glycation end products as possible is raw vegetables, steamed porridges, boiled meat and fish, fermented dairy products.
The higher the processing temperature of the products (including in the oven and in the pressure cooker), the more glycation end products are in the finished dish.
Excessive physical activity stimulates fibrosis of the heart. For example, in 2012, the research Institute at the hospital in Kansas City (USA) conducted a study among marathon runners. Half of the men were found to have cardiac fibrosis on MRI, which proceeded without symptoms. 47 marathon runners showed aortic stiffness. All these are indicators of collagen growth within the extracellular matrix with its subsequent compaction and loss of elasticity.
Sedentary lifestyle causes premature aging. But overly active, too. And the truth, as always, is somewhere in the middle. In every case, a measure is important.
Improving the functioning of the lymphatic system allows you to purify the extracellular matrix. The customs function of the extracellular matrix was discovered in 1966 by a Russian scientist in the field of medicine, biophysics and ecology – V.P. Kaznacheev, MD. He discovered that the extracellular matrix doses the transport of nutrients and passes only what is needed. He keeps the rest in himself and sends it with water to the lymphatic capillaries.
But if microcirculation is disrupted, then “toxins” slagger the extracellular matrix. Water stagnates in the tissues due to insufficient production of lymph and its outflow. Externally, we assess this condition as edema.
That is why it is so important to regularly “disperse the lymph”, perform lymphatic drainage exercises and techniques. All this helps to purify the extracellular matrix and thereby rejuvenate the body.
Aromatherapy. In 2003, an in vitro study (under artificial conditions) was conducted, which proved that certain essential oils are able to suppress the activity of elastase.
Elastase is an enzyme that destroys collagen and elastin fibers.
With age, the activity of this enzyme increases. And in order to prevent further enhanced destruction of fibers, aromatherapy with essential oils will help:
– citrus fruits (especially in combination, but you can also use lemon and grapefruit oil separately);
– juniper;
– black pepper.
Antioxidant therapy. In simpler terms, antioxidants are molecules that prevent oxidation. Including in the extracellular matrix. Antioxidants accelerate the circulation of nutrients and improve metabolism. Antioxidants also fight free radicals – molecules that have lost electrons and begin to attack neighboring molecules.
The oxidation of the body is sometimes called biological corrosion. Antioxidants are used to stop it.
Antioxidant therapy is possible with:
– Aromatherapy with essential oils (yarrow, frankincense, ylang-ylang, lemon, myrrh, marjoram, basil, rosemary, thyme);
– Ingestion of vegetable oils. Each vegetable oil contains vitamin E (tocopherol), which has antioxidant properties. Unrefined cold-pressed oils are the most valuable, because antioxidants are partially destroyed during heat treatment.
The richest sources are jojoba oil, hazelnut, sunflower, sesame, pumpkin seeds, sea buckthorn, avocado.
It is proved that in the young extracellular matrix, old cells are rejuvenated. And vice versa: in the old extracellular matrix, even young cells age.
Unfortunately, there is no way to rejuvenate the matrix itself yet. But there are options that slow down its aging, preventing the loss of its elasticity. And all this is quite possible for a person who has decided to monitor his health and counts on active longevity.
About the common truths:
– how important it is to pay attention to useful physical activities every day (especially walking);
– to be outdoors more often (oxygen enrichment is also an antioxidant therapy);
– adhere to a healthy daily routine (only a night’s sleep provides us with the rejuvenating effect of melatonin).
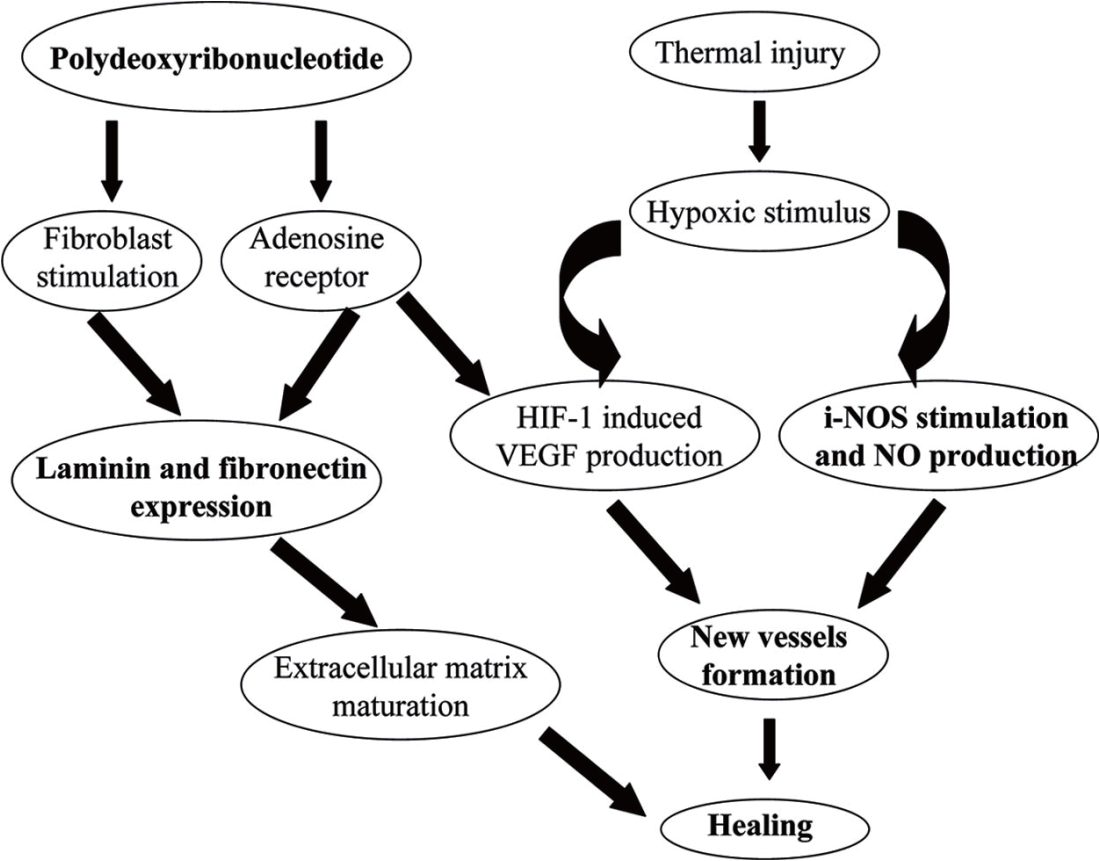
This time we would like to talk about the types of PDRN/PN products available in the market and the practical clinical content of cosmetic or anti-aging treatments performed by cosmetic medicine doctors according to the documents associated with the clinic.
PDRN is a 50–2000 base deoxyribonucleotide polymer in which purines and pyrimidine nucleotides extracted from wild salmon (oncorhynchus keta/oncorhynchus mykiss) testes/sperm are linked through an inactivation process by phosphodiester bonds.
It is a renewable substance (DNA fragments) that promotes rapid tissue regeneration in cases of excessive inflammatory reactions or tissue damage.
Based on this mechanism of action of PDRN, longer DNA fragments with higher molecular weight can be thickened into three-dimensional porous scaffolds – PN.
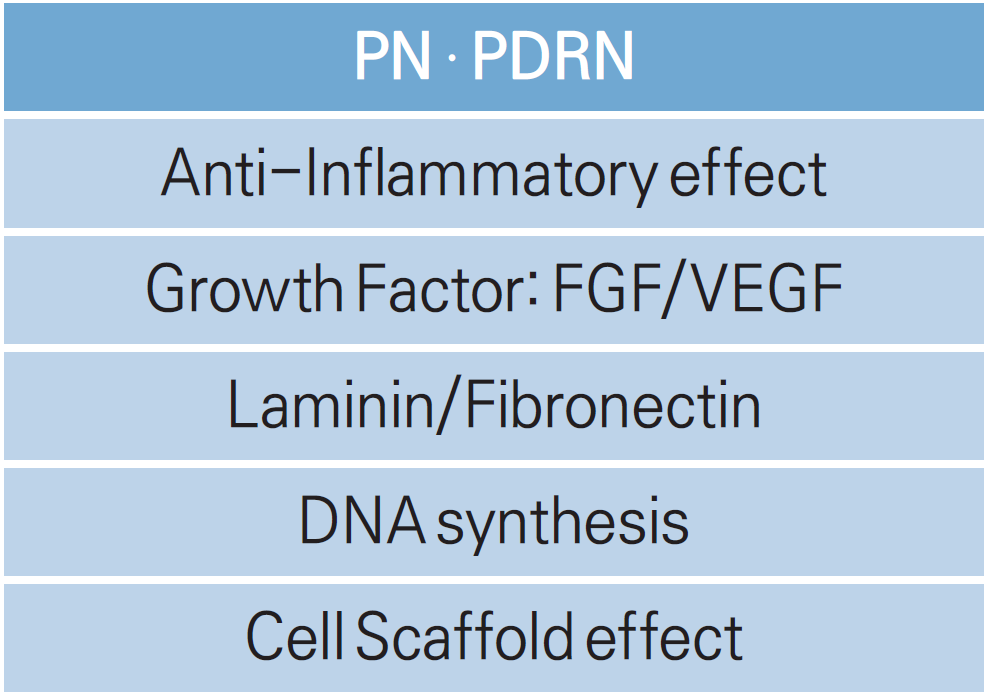
Products containing PDRN/PN
Current products on the Japanese market containing PDRN/PN as main ingredients can be divided into two categories: injectable (drugs and medical devices) and non-injectable (cosmetics).
Or they can be divided into products in which PDRN/PN is used as the sole drug, or products in which PDRN/PN is mixed with other skin-enhancing ingredients.
All of these products cause the following:
① anti-inflammatory effect
② FGF/VEGF stimulation
③ laminin/fibronectin production
④ DNA synthesis
due to the dual action of PDRN’s primary action on A2A receptors and bi-directional action along the salvage pathway, improving various clinical indications from a cosmetic and anti-aging perspective by harnessing the core effects of tissue regeneration and wound healing.
One of the best companies that produces injection products including PDRN/PN is the Japanese company Lacerta Tokyo Co.,Ltd. On the market since 2001, it produces both high-quality raw materials and high-quality products that have no equal on the Japanese market.
Product lines from Lacerta Tokyo are applicable to a variety of skin types and patient clinical conditions.
Unlike the aforementioned PDRN/PN product lines, products approved as cosmetics cannot be injected into the skin; they should be penetrated into the dermis using microneedles such as MTS and dermarollers, fractional laser or various transdermal delivery systems (TDS) such as iontophoresis, electrophoresis and sonophoresis.
Production and action of laminin and fibronectin
Fibroblasts are produced through FGF stimulation through the primary mechanism of action of PDRN/PN to regenerate collagen.
Although the fundamental principle is that the skin environment is regenerated through neovascularization with VEGF/angiopoietin, to promote healthy skin and suppress aging, we focus on the production of laminin and fibronectin.
Many studies have already demonstrated that the expression of laminin, the most important component of the basement membrane (BM), which forms the boundary and connection between the epidermis and dermis, is increased by PDRN.
Laminin is a cruciform protein located in the dense lamina CM, a bridge-like substance that plays a significant role in the interactions of the epidermis and dermis (paracrine network) and is involved in the actions of a number of cells.
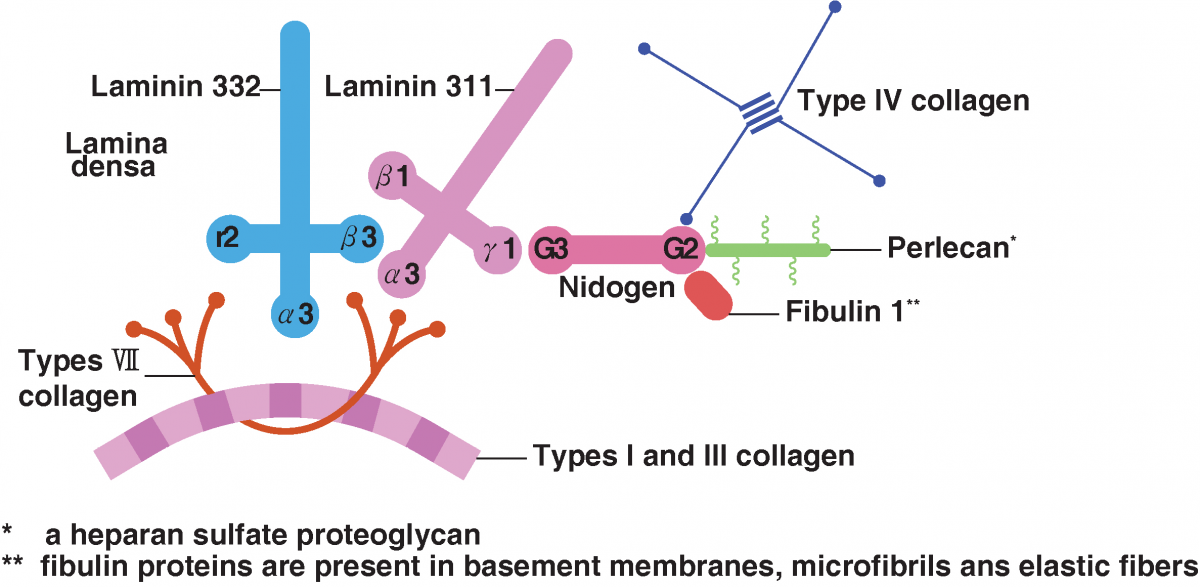
Epidermal keratinocytes, laminin, collagen types IV and VII, and collagen types I and III of the upper papillary dermis are closely associated with participation in the growth and regeneration of the epidermis and dermis.
Thus, PDRN/PN induce anti-inflammatory effects, angiogenesis, dermal regeneration through collagen regeneration, and the production of laminin and fibronectin, important components of BM, strengthening the three-step epidermis-BM-dermis connection.
Laminin and fibronectin in wound healing
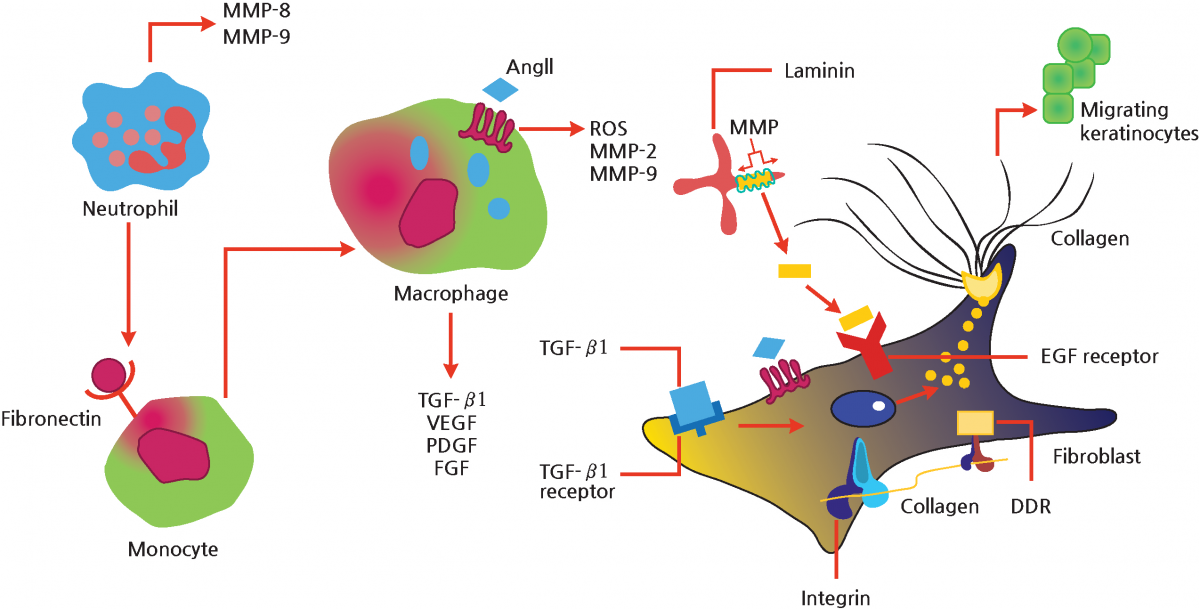
When the skin is injured, monocytes move to the site and react with fibronectin secreted by neutrophils, differentiating into macrophages, which secrete TGF-ß1, VEGF, PDGF and FGF, which play an important role in wound healing.
Meanwhile, matrix metalloproteinases (MMPs) fragment laminin, which binds to the ECF receptors of fibroblasts, then participates in the migration and proliferation of keratinocytes.
Thus, keratinocytes, fibroblasts, laminin and fibronectin are closely associated with each other, and during their processes, various growth factors induce repair and regeneration of the extracellular matrix (ECM).
In addition, laminin and fibronectin play a significant role in wound healing.
Figure 4 schematically illustrates the interactions of ECM, growth factors, and MMPs in wound site regeneration, demonstrating the important actions of laminin and fibronectin in wound healing.
Skin aging is essentially chronic damage to the dermis from ultraviolet rays.
The various anti-aging treatments we perform in our outpatient clinics using energy-based devices (EBD), laser/light, radiofrequency, high-focus ultrasound also target temporary damage to the dermis using heat.
When the damaged area is regenerated, collagen is synthesized, causing rejuvenation.
In this context, skin boosters, especially PDRN/PN, may play an important role in the wound healing process.
In conclusion, PDRN/PN promote the regeneration of various intradermal components such as collagen, laminin and fibronectin to make the ECM strong and healthy again, which can lead to improved sensitive skin, anti-aging effects and cosmetic improvements. .
In the next issue, I would like to describe the relationship of PDRN/PN to real-world clinical situations, focusing on studies with greater clinical utility.
Regulatory peptides (RP) are biologically active substances consisting of a sequential chain of amino acid residues and performing multiple polyfunctional roles in the human and animal body. The same molecule, depending on the tissues and organs, can be a neurotransmitter, neuromodulator, neurohormone and local hormone. Compared to other intercellular signaling systems, the peptide system turned out to be the most polynomial (over a thousand natural RPs have already been discovered, they form more than 40 families) and polyfunctional, and the functions of the peptides often overlap.
The system of regulatory peptides forms the so-called functional continuum (functionally continuous system). In addition to providing a wide variety of complexes of biological activity, the peptide continuum performs another function – the formation of complex regulatory chains. Each of the RPs has the ability to induce the release of certain RPs into the blood, cerebrospinal fluid, and intercellular environments of the body. A special case of this system of mutual induction is the effect of liberins and statins of the hypothalamus on the output of pituitary hormones. Each RP whose output is induced by another peptide, in turn, can induce the output of the next RP – so that a cascade of regulatory processes arises. It is now difficult to judge how long such a chain could be. It is known, however, that many RPs, the half-life of which is measured in minutes, are capable of causing multi-hour and even multi-day effects after administration to the body. Probably, the basis for this is such chains of regulatory processes. The biological meaning of the existence of long-term regulatory processes consisting of short-term stages is obvious. Unlike systems based on long-lived regulators, such a system is more flexible in a changing situation, when new signals arrive, etc.
The basic law of the orderly regulatory mission of the Republic of Poland is the principle “What? Where? When?” (What? – what peptide? Where? – in what locus of the body does its expression take place? When? – when does its synthesis occur?). This principle is achieved due to the ability of peptides to optimally and mobilely combine the synthesis and/or release of the corresponding peptide in the right place and at the right time.
Structure of PIL Peptides are built as combinations of amino acids. There are 3 categories of regulatory peptides. Signs that determine whether a peptide belongs to one category or another are: amino acid composition, half-life, lifespan, degree of affinity for receptors, the presence or absence of a specialized precursor protein, the presence or absence of a transporter protein, the presence of a producing organ for this peptide.
Category I of RP – classical – includes peptides of a distant type of action with a low half-life and high affinity for receptors. These are small and medium-sized peptides – from 2 to 60 amino acid residues, they have a specialized precursor protein, as well as a special carrier protein. Most peptides in this category have a producing organ.
RP of category II were discovered relatively recently – these are glyprolines, small peptides, the number of amino acids from 2 to 7, high proline content. This also includes some exorphins. Representatives of this category have a very high half-life, low affinity for receptors, and a non-specialized precursor protein (collagen, elastin). Peptides have a distant effect on the blood system and the mucous membrane of the stomach and intestines.
Category III RPs have low affinity for receptors, do not have a specialized precursor protein, and the number of amino acids ranges from 2 to 60. Representatives of this group are classified as tissue-specific peptides of local significance. Due to their low half-life, their distant action is impossible.
Biosynthesis (processing) of regulatory peptides. The biochemical “kitchen” of peptide synthesis turns out to be the same for all body systems. The general principle of the biosynthesis of all sufficiently studied RPs is the formation of relatively large precursor peptides, from which, after completion of translation, the corresponding RPs are cleaved by proteinases. As a rule, the precursor peptide includes several main sequences and a so-called signal sequence that promotes the migration of the precursor inside the cell after completion of translation in the rough endoplasmic reticulum (the first few minutes) and is cleaved off at the end of this part of the path. Many precursor peptides form intermediates with glycosides, which also have a stabilizing effect at some stages of processing and influence the selection of sites of attack by proteinases.
Proteinases that ensure the cleavage of NPs within several tens of minutes or an hour after translation (in the Golgi complex, during movement along the axon and during the formation of vesicles) are not highly specific. To a large extent, accurate cleavage is determined by the presence of peptides in the amino acid sequence – precursors of paired residues. They are primarily attacked by proteinases similar in their action to cathepsin B and trypsin. The fragments eliminated during this processing phase in many cases already represent active RP. In some cases, these fragments undergo further proteolysis again with the formation of new RPs. At this phase, the participation of not only trypsin-like, but also a number of other proteinases, the specificity of which is also not very high, has been described.
Reception of regulatory peptides. RPs interact with receptors, which are specialized membrane structures where the information signal is converted into a physiological act. At present, there is no doubt about the existence of specialized isolated receptors for regulatory peptides. In addition, membrane molecular formations that specifically bind RP are, in some cases, part of classical neurotransmitter receptors. An example of this is the GABAA receptors, which are receptors for endozepines.
Physiological processes that occur in a cell after contact of the RP with its receptors on its membrane cause activation of the system of second messengers. In this case, some peptides can induce the formation of only one type of second messengers, while others can change the content of several types depending on the type of receptors.
Neuropeptides (NPs) are universal peptide regulators that occupy an important place in the chemical transmission of information, primarily in the nerve cell. The NP category usually includes small and medium-sized peptides – from 2 to 50-60 amino acid residues. Larger peptides, which include a number of hormones, some cell growth factors and a number of other factors, usually contain over 100 amino acid residues, and they are usually classified as regulatory proteins. Most NPs are linear peptides. The C-terminal amino acids in them are often amidated, and the N-terminal glutamic acid residues are often represented by pyroglutamate. Other modifications of a/c residues are rare. NPs are formed as a result of limited proteolysis of large precursor peptides and are secreted using mechanisms similar to those known for non-peptide mediators.
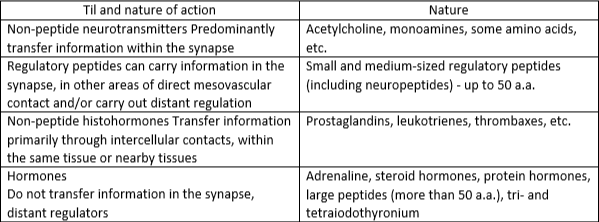
Localization of NP. NPs are widely represented in the brain and peripheral nervous system. The content of peptides varies between 10-12-10-9 M. In addition, in a number of organs and tissues, peptides that are identical or similar to many NPs are synthesized and secreted by non-nerve cells. This forces us to consider NPs as part of the body’s general system of regulatory peptides. At the same time, some terminological difficulties arise. For example, peptides produced in endocrine glands, cardiomyocytes, gastrointestinal tract cells and immune systems can influence the activity of the peripheral and central nervous system. Although they are not of neuronal origin, apparently they should be classified as NP, especially since almost all carefully conducted experiments in the central nervous system also reveal neurons that synthesize a peptide identical to that found in the periphery (Table 1).
Table 1
The place of neuroiptides among other substances that carry out intercellular information transfer
Features of peptidergic transmission. Peptidergic neurons are widely represented in the central and metasympathetic nervous systems. The peptidergic neuron is fundamentally similar to any other neuron and has biochemical and physiological mechanisms for the synthesis, deposition, and secretion of the peptide mediator. The peptidergic synapse is also not fundamentally different from the synapse of any other chemical nature. However, often the peptide is released from the ending of a peptidergic neuron not in the synapse, but directly into glia or into the blood and performs the function of an extrasynaptic modulator or neurohormone. In addition, peptides can be released not only from the terminals of peptidergic neurons, but also from the terminals of other, non-peptidergic neurons. In this case, the peptide acts as a synaptic modulator (cotransmitter, comodulator).
However, the following features are distinguished for peptidergic transmission;
1. NPs exist for quite a long time in biological fluids of the body – from 10 ms to 1-2 hours, which determines the possibility of their long-term regulatory effect on the cell.
2. All NPs have a common biosynthetic precursor.
3. Biosynthesis of NP is carried out by its cleavage by proteases from the precursor peptide.
4. NP breakdown products are biologically active compounds; they have their own spectrum of biological activity, often opposite to the spectrum of activity of their predecessor, i.e. a transformation of their activity is observed.
5. A characteristic feature of peptidergic transmission is the multiplicity of neuropeptides. Their number is so large (> 50), which makes it possible for their endless combination and mutual influence.
6. After secretion of NP into the synaptic cleft and interaction with postsynaptic receptors, they are not reabsorbed by the presynaptic membrane or glial cell.
Processing of neuropeptides. Processing of neuropeptides consists of the formation of relatively large inactive precursor peptides, from which, after completion of translation, the corresponding NPs are cleaved by proteinases. This process takes place on the rough ER and is strictly controlled by the cell genome.
The precursor peptide contains one or more basic (or trigger) amino acid sequences. They can differ significantly from each other in structure and belong to different classes of neuropeptides. In addition, the peptidide precursor molecule necessarily includes several signal sequences that serve as a site of cleavage by proteinases or promote migration of the precursor to the axon terminal of the peptidergic neuron. The signal sequences are as follows: arginine-arginine, lysine-lysine, lysine-arginine, arginine-lysine. The precursor peptide may contain up to 200-300 amino acid sequences. In addition to the latter, its molecule includes glycosidic cations, which temporarily bind to it, exerting a stabilizing effect on the molecule during its transport to the terminal and determine the sites of attack by proteinases.
Several precursors for neuropeptide synthesis are known, but only three have been well studied. They are the main sources of endorphins and enkephalins. These are proopiomelanocortin, proenkephalin A, proenkephalin B.
Proopiomelanocortin has 265 amino acid sequences and contains beta-endorphin, ACTH, alpha, beta, gamma melanocyte-stimulating hormone. Its minimal trigger sequence is methenkephalin. Its synthesis is localized in the cells of the anterior and intermediate lobes of the pituitary gland, hypothalamus and some other areas of the brain, as well as in the gastrointestinal tract, placenta and lungs. From this precursor ACTH1-39 is formed; ACTH 1-24; ACTH 18-39, alpha, beta, gamma melanocyte-stimulating hormones, beta, gamma and alpha endorphins, metenkephalin and some other lesser known neuropeptides.
Proenkephalin A consists of 263 amino acid sequences. The trigger sequence is leu-enkephalin. This precursor represents the main source of various enkephalins.
Proenkephalin B consists of 256 amino acid sequences, the main source of endorphins. The minimal trigger sequence is leu-enkephalin.
In addition, it has been shown that neuroactive products can be formed during the breakdown of albumin, immunoglobulin G, i.e. these bioorganic molecules can be considered as precursors of neuropeptides and other regulatory peptides.
It must be emphasized that all neuropeptides are not formed simultaneously in one neuron or one terminal, but selectively depending on the set of proteinases in a given neuron or nerve terminal.
Pathways of neuropeptide inactivation. Not studied enough. It is known that;
1. Often, enzymes that cleave the peptide – aminopeptidases, carboxypeptidases, are associated with peptide receptors, which can be located on the postsynaptic membrane or located extrasynaptically (for example, on smooth muscle cells, blood cells).
2. The complex formed during the interaction of the peptide with the receptor undergoes internalization into the cell cytosol and is transported
1) either into the lysosome and there the peptide is cleaved by aminopeptidases and kgfboxypeptidases;
2) or affects the cytosolic receptor.
3. Degradation enzymes can be common to many peptides at one stage, and extremely specific at some stages. Moreover, sometimes enzymes that metabolize classical mediators may be involved in degradation. For example, for substance P, such an enzyme could be cholinesterase.
4. Very often, for many peptides in the body, the distributions of the maximum densities of the enzyme systems of synthesis and degradation, the peptides themselves and their receptors do not coincide, which indicates that all these processes are observed in different areas.
5. Peptide degradation occurs quite quickly to amino acids. Exogenous enkephalin is broken down in dog and human plasma within 2 minutes, melanocyte-stimulating hormone – 1.6 minutes, ACTH – 2-5 minutes, substance P – 3-4 minutes.
6. Many authors note the species-specific nature of the rate of cleavage of the same peptide.
7. It was noticed that the rates of peptide breakdown in vivo and in vitro do not coincide. Some peptides (alpha-melanocyte-stimulating hormone) are not cleaved in vitro.
8. Many researchers note that some exogenous and endogenous peptides (the same ones) can be broken down by different enzyme systems. Neuropeptide receptors. The physiological effect of neuropeptides is realized through membrane and cytosolic receptors. They are highly specific. Each neuropeptide has its own receptor, and sometimes two. Neuropeptide receptors are:
– as independent protein-lipid complexes, where the lipid plays no less significant role than the protein;
– and combined receptors, sometimes representing part of the receptor for classical neurotransmitters;
– conformationally mobile structures;
– capable of clustering. On the surface of the membrane in the so-called receptor pit, they are grouped in groups of 2-12 molecules together in complex with the glycocalex and can be internalized into the cytosol to interact with cytosolic receptors;
– most neuropeptides in solution are conformationally mobile structures and their interaction with the receptor is best explained based on the concept of a “dynamic pharmacophore” or mutually induced correspondence between the ligand and the receptor;
– all known mechanisms can be used as coupling mechanisms. Often one receptor is associated with two mechanisms simultaneously.
Relationship between the neuropeptidergic system and classical neurotransmitters. It is now quite definitely established that:
– neuropeptides and amines can be synthesized and stored in one nerve cell;
– deposition of neuropeptides and classical mediators occurs either in the same vesicles or in different ones.
In connection with these data, a concept was formed about the existence in the mammalian body of a diffuse neuroendocrine system, the cells of which simultaneously produce monoamines and neuropeptides. This system in the Anglo-American literature is called “APUD” (amino precursor uptake and decarboxylation). This system is characterized by:
1. Firstly, a common embryological origin from a single predecessor. Cells of the “APUD” system are distributed throughout the central nervous system and somatic organs. They produce monoamines and more than 40 physiologically active peptides, 15 of which have a common localization in the brain and gastrointestinal tract.
2. Phylogenetically, neuropeptides are more ancient regulators, despite their complex structure compared to classical neurotransmitters. And in fact, the evolutionary path of the formation of a new neuroactive oligopeptide sequence turns out to be much shorter than the formation of a non-peptide mediator. For example, the phylogenetic formation of norepinephrine from tyrosine requires the emergence of three new enzymes (DOPA decarboxylase, tyrosine hydroxylase and DA beta-hydroxylase) based on changes in three genes. The formation of a peptide sequence of the ACTH4-7 type requires only a small number of miss mutations in a limited region of one gene.
3. The commonality of neuropeptides and monoamines is also manifested in the structure of the active center of monoamine receptors and peptide regulators. It consists of an aromatic moiety and a (+) charge, which is a possible reason why peptides interact with some monoamine receptors.
Functional significance of neuropeptides. Many of the NPs function as neurotransmitters that transmit signals within the synapse. In this case, NPs, as a rule, “cooperate” with non-peptide mediators; in the same nerve ending, certain combinations of a non-peptide neurotransmitter with one, two, and sometimes three NPs are noted. Depending on the frequency and duration of the impulses, they are distinguished jointly or separately. Sometimes such NPs are called koneirotransmitters or koneirotransmitters. It should be noted that the biochemical properties of NP determine the functional differences from classical mediators. The most obvious are the following:
1. Long duration of existence of NPs in body fluids, especially NPs of medium and large size with the number of amino acid residues exceeding 14-20; this determines the duration of intrasynaptic modulation and the possibility of distant action on distant synaptic systems.
2. The presence of a certain, often high and specific activity in the products of incomplete proteolysis of NP; proteolysis of a number of peptides is considered not as simple inactivation, but as a transformation of their activity.
3. A large number of different NPs, far exceeding the number of conventional neurotransmitters; this creates not only quantitative, but also qualitatively new possibilities for the interconnection of various NPs and neurotransmitters within one neuron, one terminal.
In addition to participating in signal transmission at the synapse, NPs are capable of transmitting information over longer distances – in small areas, in an organ, and even within the whole organism. In this case, their functions are indistinguishable from the function of hormones (including histohormones). The objects of the distant action of NPs are the pre- and post-synaptic zones of neurons, as well as other cells. At the same time, NPs can facilitate or inhibit impulse transmission and have other effects on the state of the neuron, i.e. function as neuromodulators. In different parts and subsections of the brain, in different neurons, the same NPs can perform either neurotransmitter (coneirotransmitter) or distant neuromodulatory functions, and sometimes combine them.
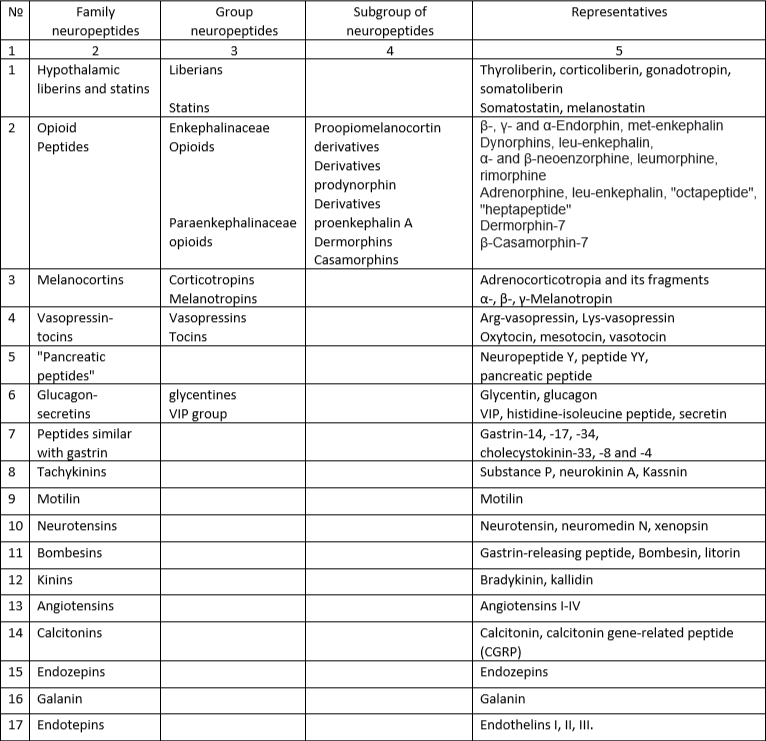
The modern classification of NP is based on a combination of three principles: functional, structural and topological (based on the place of synthesis) (see Table 1).
Cocalcigenin – CGRS (CALCITONIN GENE RELATED PEPTIDES). The biological activity of cocalcigenin is manifested very clearly – the introduction of only ≈10-14 moles of this NP under the human skin causes a rather long-term dilation of arterioles, increased blood flow and (especially together with substance P) diapedesis from blood vessels. Together with VIP and Y-peptide, cocalcigenin is involved in the regulation of vascular tone in the brain, coronary vessels, etc. Atriopeptides. Until recently, they were considered factors of purely peripheral origin and action. Their main source is atrial cardiomyocytes.
Atriopeptides significantly increase diuresis and especially sodium uresis. Now, however, it has been proven that atriopeptides are also synthesized in the brain. Their functions in the central nervous system have so far been poorly studied.
Endozepins. They are negative regulators of GABA receptors. If GABA itself serves as an inhibitory mediator involved in various processes, including tranquilization – reducing feelings of anxiety, fear, restlessness, then endozepins, on the contrary, cause restlessness, anxiety, the appearance of fear and, in experiments on rodents, pro-conflict behavior. Apparently, the suppression of GABAergic transmission, which is very widely represented in the brain, suggests other manifestations of biological activity in endozepins, but studies of this group of NPs began relatively recently. It is known that endozepine consists of 100 amino acid residues, its active fragments are small 18- and 6-membered peptides. Receptors for them are part of the GABA receptors (GABA receptors) or independent receptors tightly associated with the GABA receptor.
Somatostatin (SST). Somatostatin was first isolated in 1973 from the hypothalamus of cattle. Consists of 14 amino acid residues. One of the releasing factors of the hypothalamus is of the inhibitory type. It was first identified immunocytochemically in single fibers of the sympathetic nervous system along with NA. SST inhibits the release of NA. Later, it was identified in cholinergic neurons of the parasympathetic fiber of the heart (vagus), weakening the intensity of ACh release. This effect of SST on the release of classical neurotransmitters is associated with its presynaptic modulatory effect.
Table 1
Classification of neuropeptides (according to: Ashmaria I.P., 1999)
In the sympathetic pathways of the spinal cord – in P-ergic fibers, in the cerebral cortex – in GABA-ergic fibers, where SST promotes the release of substance P and GABA.
In the human body, SST exists in 2 biological forms: linear and cyclic. Predecessor – SST-28 => SST-14. In different tissues, CCT-28 and CCT-14 are found in different ratios. The precursor of CCT-28 and CCT-14 is prosomatostatin-140 (consists of 140 amino acid residues). During the processing of prosomatostatin, 4 cleavage sites are possible and 7 somatic-like peptidodes with different molecular weights are formed, but biological activity was detected only for CCT-28 and CCT-14.
Localization. 75% of somatostatin is localized in the digestive tract and 25% in the brain. The greatest activity of SST is in the hypothalamus, stomach, intestines and pancreas, where it is identified in SST-containing neurons of the central nervous system and metasympathetic nervous system. Their lower density is in the limbic system and cortex, basal ganglia, pituitary gland, hippocampus, amygdala, and projection neurons of the brain stem.
SST-containing neurons are also present in specialized sensory systems – olfactory (olfactory bulb), visual (retina), auditory (internal geniculate body).
Somatostatin is also present in endocrine D-cells of the gastric mucosa (antrum), pancreas, and thyroid gland.
The half-life of CCT-14 is 2-4 minutes, which is longer than other peptides.
Receptors and coupling mechanisms. SST exerts its effect on the cell through somatostatin receptors (SST). There are 5 types (SSTi,2,3,4,5) – All types of receptors are associated with G-proteins. A gene has been identified in rats that controls the synthesis of this receptor. The molecular structure of the receptor has been deciphered. The receptor is an oligopeptide of 7 transmembrane domains. In the CNS, the receptor density is distributed in descending order as follows; cortex, thalamus, hypothalamus, striatum, medulla oblongata, spinal cord (dorsal horns, central gray matter).
The SST1 – receptor is associated with AC through SST1 proteins, i.e. SST lowers the level of cAMP in the cell. This is the most common type of receptor, but its maximum density is found in the hypothalamus. In many regions of the brain, excitation of this type of receptor is accompanied by inhibition of the impulse activity of neurons. Both forms of somatostatin bind to this type of receptor, but CCT-14 has a slightly higher affinity. SST1 receptor antagonists of synthetic origin are known.
SST2 – controls the activity of Ca^* and lC channels, inhibiting their activation. The highest density of this receptor is found on neurons of the locus coeruleus, pituitary gland and on cells of the gastric mucosa. Through this type of receptor, the universal inhibitory properties of CCT-14 are manifested. CCT-14 inhibits the impulse activity of locus coeruleus neurons, the synthesis of growth hormone, inhibits the onset of the paradoxical phase of sleep, and the production of hydrochloric acid. Synthetic antagonists are available.
SST3 – divided into subtypes A and B. Subtype A is associated with AC through Gi proteins. Subtype B is associated with FLS through Gs proteins. It is found in many structures of the brain, in the muscle layer of the stomach, where it controls the motility of the stomach and intestines, weakening it.
SST4 – is associated with AC through Gi proteins. Agonists of synthetic origin are known, but their affinity is 10 times less than that of natural somatostatin. The highest density of these receptors is found in the retina, placenta, and brain. Their important role in recognizing a visual image and in regulating the activity of the placenta has been established.
SST5 – receptor has 2 subtypes: the first type is associated with adenylate cyclase through Gi proteins; the second type is associated with FLS C through Gs proteins. SST, through these receptors, inhibits the release of pituitary hormone into the blood and insulin from the pancreas, in addition, it has an antiproliferative effect in vascular smooth muscles, i.e. inhibits fibrous growth of the vessel wall and a decrease in its lumen in connection with this.
Thus, through receptors type 2 and 5, CCT-14 and CCT-28 inhibit the secretion of pituitary hormones (ACTH, growth hormone, prolactin, thyrotropin and gonadotropin). Through receptors type 1 and 5, it inhibits the release of glucagon and insulin into the blood.

Lacerta Tokyo Сo.,Ltd.
Privacy policy
Refusal of unauthorized collection of email
Copyright © 2016 LACERTA, Inc. All rights reserved.